232
Dow Hall, Dept. Physics |
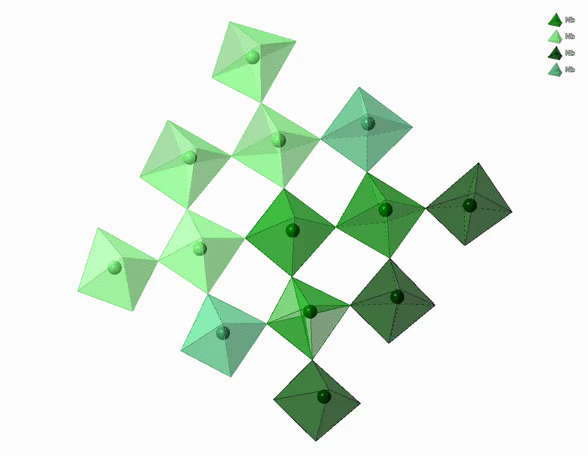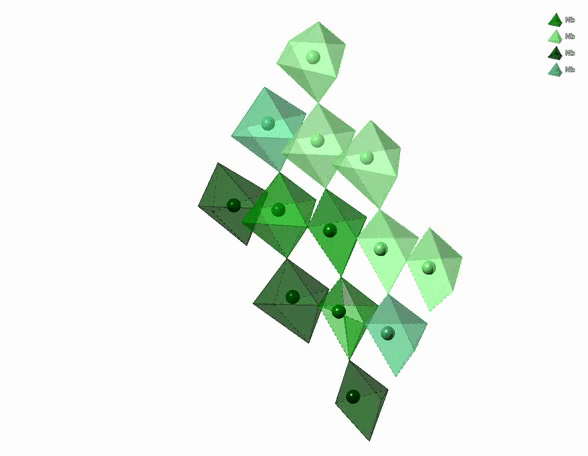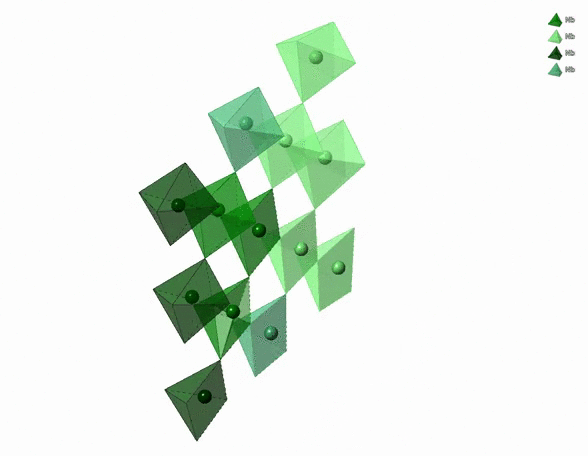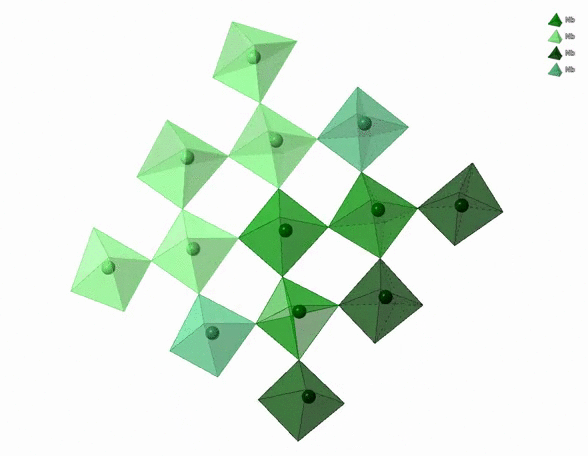
The physico-chemical properties of materials are highly dependent on the 3D arrangement of atoms that build them. For example, diamonds are hard, transparent and expensive. Graphite is black, soft and cheap. Both are pure carbon though. The difference comes from the distinct 3D arrangement of carbon atoms in diamonds and graphite. In particular, carbon atoms in diamonds form a tetrahedral network whereas carbon atoms in graphite form a stack of hexagonal layers.
Examples of our work:
V2O5 nanotubes ..............................Synthetic Polymers ............................................Perovskites ..........................................................Gas phase reactions
News:
April 2025: We conducted an experiment at NSLS II, Brookhaven
December 2024: Paper with our results from in situ x-ray scattering experiments on fuel cells appeared in JACS.
Fall 2024: For a 4th consecutive year, V. Petkov is listed among the top 2 % of world’s most-cited scientists
Fall 2024: Thanks are due to DEVCOM GVSC for the continued support of our research on battery materials.
October 2024: We conducted a successful experiment at DESY, Germany.
Fall 2024: We welcome Carlos as a grad student.
Summer 2024: We bid farewell to our postdocs Ruhul and Bruno.
March 2024: We conducted a succesful experiment at NSLS II/Brookhaven
|
| _Highlights_ | _Journal_covers_ | _Papers_ | _Xray_Lab_ | _Teaching_ | _Software_ | _People_ |