51V-Vanadium
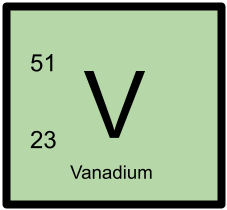 51V has 23 protons and 28 neutrons.
51V is a stable isotope.
The mass excess of 51V is -52203.105 with an uncertainty of ±0.097.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:
51V has 23 protons and 28 neutrons.
51V is a stable isotope.
The mass excess of 51V is -52203.105 with an uncertainty of ±0.097.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:
Influence Equations
58% 51V-48Ti1.063 1
34.7% 50V(n, γ)51V 2
3.5% 51V(p, n)51Cr 3
Reference
1. R.M.E.B. Kandegedara, G. Bollen, M. Eibach, N.D. Gamage, K. Gulyuz, C. Izzo, M. Redshaw, R. Ringle, R. Sandler, A.A. Valverde (2017) β-decay Q values among the A = 50, Ti-V-Cr isobaric
triplet and atomic masses of 46,47,49,50Ti, 50,51V and 50,52-54Cr Physical Review C 96(4)
https://journals.aps.org/prc/abstract/10.1103/PhysRevC.96.044321
2. A. Robertson, T.J. Kennett, W.V. Prestwich (1978) Thermal neutron capture in 50V Zeitschrift fur Physik A 284(407-411)
https://link.springer.com/article/10.1007/BF01422109
3. C.R. Gossett, J.W. Butler (1959) Neutron Thresholds in the 51V(p, n)51Cr, 55Mn(p, n)55Fe, 70Zn(p, n)70Ga, and 75As(p, n)75Se
Physical Review 133(1)
https://journals.aps.org/pr/abstract/10.1103/PhysRev.113.246
 51V has 23 protons and 28 neutrons.
51V is a stable isotope.
The mass excess of 51V is -52203.105 with an uncertainty of ±0.097.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:
51V has 23 protons and 28 neutrons.
51V is a stable isotope.
The mass excess of 51V is -52203.105 with an uncertainty of ±0.097.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:51V has 23 protons and 28 neutrons. 51V is a stable isotope. The mass excess of 51V is -52203.105 with an uncertainty of ±0.097. The mass was measured using a Penning Trap and Nuclear Physics and the related equations include: