105Pd-Palladium
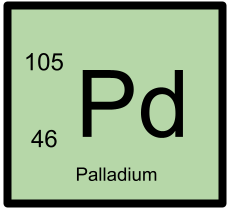 105Pd has 46 protons and 59 neutrons.
105Pd is a stable isotope.
The mass excess of 105Pd is -88417.905 with an uncertainty of ±1.138.
The mass was measured using Nuclear Physics and the related equations include:
105Pd has 46 protons and 59 neutrons.
105Pd is a stable isotope.
The mass excess of 105Pd is -88417.905 with an uncertainty of ±1.138.
The mass was measured using Nuclear Physics and the related equations include:
Influence Equations
96% 105Pd(n, γ)106Pd 1
3.9% 105Rh(β-)105Pd 2
0.2% 105Pd(3He, d)106Ag 3
Reference
1. L.M. Bollinger, G.E. Thomas (1970) Average-Resonance Method of Neturon-Capture γ-Ray Spectroscopy: States of
106Pd, 156Gd, 158Gd, 166Ho, and 168Er Physical Review C 2(5)
https://journals.aps.org/prc/abstract/10.1103/PhysRevC.2.1951
2. R.B. Duffield, L.M. Langer (1951) Radioactivities of 105Ru, 105Rh, 84Br, and 83Br
Physical Review 81(2)
https://journals.aps.org/pr/abstract/10.1103/PhysRev.81.203
3. R.E. Anderson, R.L. Bunting, J.D. Burch, S.R. Chinn, J.J. Kraushaar, R.J. Peterson, D.E. Prull, B.W. Ridley, R.A. Ristinen (1975) A study of the 106Pd(p, d)105Pd
and 106Pd(3He, d)107Ag reactions Nuclear Physics A 242(1)
https://www.sciencedirect.com/science/article/pii/0375947475900354
 105Pd has 46 protons and 59 neutrons.
105Pd is a stable isotope.
The mass excess of 105Pd is -88417.905 with an uncertainty of ±1.138.
The mass was measured using Nuclear Physics and the related equations include:
105Pd has 46 protons and 59 neutrons.
105Pd is a stable isotope.
The mass excess of 105Pd is -88417.905 with an uncertainty of ±1.138.
The mass was measured using Nuclear Physics and the related equations include:105Pd has 46 protons and 59 neutrons. 105Pd is a stable isotope. The mass excess of 105Pd is -88417.905 with an uncertainty of ±1.138. The mass was measured using Nuclear Physics and the related equations include: