16O-Oxygen
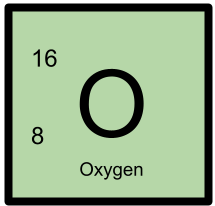 16O has 8 protons and 8 neutrons.
16O is a stable isotope.
The mass excess of 16O is -4737.00217 with an uncertainty of ±0.0003.
The mass was measured using a Penning Trap and the related equations include:
16O has 8 protons and 8 neutrons.
16O is a stable isotope.
The mass excess of 16O is -4737.00217 with an uncertainty of ±0.0003.
The mass was measured using a Penning Trap and the related equations include:
Influence Equations
50.3% C4-O3 1
18.6% 16O-H16 2
16.2% O2-31PH 3
Reference
1. "Proc. 9th Int. Conf. Atomic Masses and Fundamental Constants AMCO-9, and 6th Int. (1992)
Conf. Nuclei far from Stability NUFAST-6" R.S. Van Dyck,Jr., D.L. Farnham, P.B. Schwinberg
2. F. Heiße, S. Rau, F. Köhler-Langes, W. Quint, G. Werth, S. Sturm, and K. Blaum (2019), High-precision mass spectrometer for light ions
Physical Review A 100(2)
https://journals.aps.org/pra/abstract/10.1103/PhysRevA.100.022518
3. Redshaw, M., McDaniel, J., & Myers, E. G. (2008). Dipole moment of PH+ and the atomic masses of Si28, P31 by comparing cyclotron
frequencies of two ions simultaneously trapped in a penning trap. Physical Review Letters, 100(9), [093002].
https://doi.org/10.1103/PhysRevLett.100.093002
 16O has 8 protons and 8 neutrons.
16O is a stable isotope.
The mass excess of 16O is -4737.00217 with an uncertainty of ±0.0003.
The mass was measured using a Penning Trap and the related equations include:
16O has 8 protons and 8 neutrons.
16O is a stable isotope.
The mass excess of 16O is -4737.00217 with an uncertainty of ±0.0003.
The mass was measured using a Penning Trap and the related equations include:16O has 8 protons and 8 neutrons. 16O is a stable isotope. The mass excess of 16O is -4737.00217 with an uncertainty of ±0.0003. The mass was measured using a Penning Trap and the related equations include: