146Nd-Neodymium
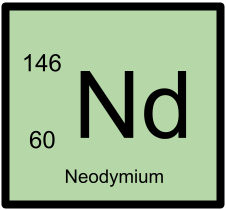 146Nd has 60 protons and 86 neutrons.
146Nd is a stable isotope.
The mass excess of 146Nd is -80925.916 with an uncertainty of ±1.273.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:
146Nd has 60 protons and 86 neutrons.
146Nd is a stable isotope.
The mass excess of 146Nd is -80925.916 with an uncertainty of ±1.273.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:
Influence Equations
84.5% 145Nd(n, γ)146Nd 1
15.5% 146Nd(n, γ)147Nd 2
1.5% 148Nd35Cl-146Nd37Cl 3
Reference
1. M.A. Islam, T.J. Kennett, W.V. Prestwich (1982) Neutron separation energies of some heavy nuclides
Physical Review C 25(6)
https://journals.aps.org/prc/abstract/10.1103/PhysRevC.25.3184
2. R. Rousille, J.A. Pinston, H. Borner, H.R. Koch, D. Heck (1975) Level structure of 147Nd (I) The 146Nd(n, γ) reactions
Nuclear Physics A 246(2)
https://www.sciencedirect.com/science/article/pii/0375947475906533
3. R.C. Barber, H.E. Duckworth, B.G. Hogg, J.D. Macdougall, W. McLatchie, P. Van Rookhuyzen (1964) The Mass Effect Corresponding to the Onset of Nuclear Deformation in the Region N ~ 90
Physical Review Letters 12(21)
https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.12.597
 146Nd has 60 protons and 86 neutrons.
146Nd is a stable isotope.
The mass excess of 146Nd is -80925.916 with an uncertainty of ±1.273.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:
146Nd has 60 protons and 86 neutrons.
146Nd is a stable isotope.
The mass excess of 146Nd is -80925.916 with an uncertainty of ±1.273.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:146Nd has 60 protons and 86 neutrons. 146Nd is a stable isotope. The mass excess of 146Nd is -80925.916 with an uncertainty of ±1.273. The mass was measured using a Penning Trap and Nuclear Physics and the related equations include: