143Nd-Neodymium
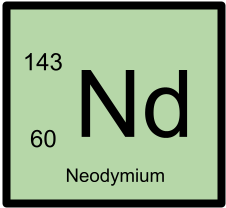 143Nd has 60 protons and 83 neutrons.
143Nd is a stable isotope.
The mass excess of 143Nd is -84002.31 with an uncertainty of ±1.255.
The mass was measured using Nuclear Physics and the related equations include:
143Nd has 60 protons and 83 neutrons.
143Nd is a stable isotope.
The mass excess of 143Nd is -84002.31 with an uncertainty of ±1.255.
The mass was measured using Nuclear Physics and the related equations include:
Influence Equations
60.9% 143Nd(n, γ)144Nd 1
22.5% 142Nd(n, γ)143Nd 1
12.8% 176Lu37Cl-143Nd35Cl2 2
Reference
1. M.A. Islam, T.J. Kennett, W.V. Prestwich (1982) Neutron separation energies of some heavy nuclides
Physical Review C 25(6)
https://journals.aps.org/prc/abstract/10.1103/PhysRevC.25.3184
2. F.C.G. Southon, J.O. Meredith, R.C. Barber, H.E. Duckworth (1977) Precise atomic mass differences for wide doublets as determined with the Manitoba II mass spectrometer
Canadian Journal of Physics
https://cdnsciencepub.com/doi/abs/10.1139/p77-053
 143Nd has 60 protons and 83 neutrons.
143Nd is a stable isotope.
The mass excess of 143Nd is -84002.31 with an uncertainty of ±1.255.
The mass was measured using Nuclear Physics and the related equations include:
143Nd has 60 protons and 83 neutrons.
143Nd is a stable isotope.
The mass excess of 143Nd is -84002.31 with an uncertainty of ±1.255.
The mass was measured using Nuclear Physics and the related equations include:143Nd has 60 protons and 83 neutrons. 143Nd is a stable isotope. The mass excess of 143Nd is -84002.31 with an uncertainty of ±1.255. The mass was measured using Nuclear Physics and the related equations include: