15N-Nitrogen
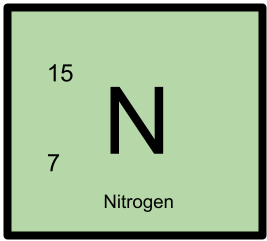 15N has 7 protons and 8 neutrons.
15N is a stable isotope.
The mass excess of 15N is 101.43809 with an uncertainty of ±0.00058.
The mass was measured using a Penning Trap and the related equations include:
15N has 7 protons and 8 neutrons.
15N is a stable isotope.
The mass excess of 15N is 101.43809 with an uncertainty of ±0.00058.
The mass was measured using a Penning Trap and the related equations include:
Influence Equations
60.9% CDH-15N 1
25.6% 15N2-28SiH2 1
13.5% CH3-15N 1
Reference
1. F DiFilippo, V Natarajan, M Bradley, F Palmer, D E Pritchard (1995), Accurate atomic mass measurements from Penning trap mass comparisons of individual ions
Physica Scripta 1995(144)
https://iopscience.iop.org/article/10.1088/0031-8949/1995/T59/018
 15N has 7 protons and 8 neutrons.
15N is a stable isotope.
The mass excess of 15N is 101.43809 with an uncertainty of ±0.00058.
The mass was measured using a Penning Trap and the related equations include:
15N has 7 protons and 8 neutrons.
15N is a stable isotope.
The mass excess of 15N is 101.43809 with an uncertainty of ±0.00058.
The mass was measured using a Penning Trap and the related equations include:15N has 7 protons and 8 neutrons. 15N is a stable isotope. The mass excess of 15N is 101.43809 with an uncertainty of ±0.00058. The mass was measured using a Penning Trap and the related equations include: