25Mg-Magnesium
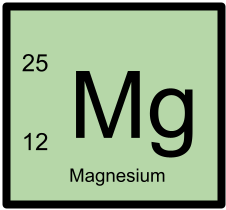 25Mg has 12 protons and 13 neutrons.
25Mg is a stable isotope.
The mass excess of 25Mg is -13192.782 with an uncertainty of ±0.047.
The mass was measured using Nuclear Physics and the related equations include:
25Mg has 12 protons and 13 neutrons.
25Mg is a stable isotope.
The mass excess of 25Mg is -13192.782 with an uncertainty of ±0.047.
The mass was measured using Nuclear Physics and the related equations include:
Influence Equations
45.9% 25Mg(n, γ)26Mg 1
42.7% 24Mg(n, γ)25Mg 2
11.4% 25Mg(p, γ)26Al 3
Reference
1. M.A. Islam, T.J. Kennett, S.A. Kerr, and W.V. Prestwich (1980) A self-consistent set of neutron separation energies
Canadian Journal of Physics
https://doi.org/10.1139/p80-028
2. 1969 Proc. Conf, Neutron Capture Gamma Ray Spectroscopy R. Hardell
3. E.O.De Neijs, M.A. Meyer, J.P.L. Reinecke, D.Reitmann (1974), The energy levels of 26Al
Nuclear Physics A 230(3)
https://doi.org/10.1016/0375-9474(74)90152-3
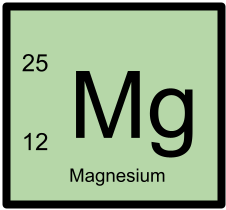 25Mg has 12 protons and 13 neutrons.
25Mg is a stable isotope.
The mass excess of 25Mg is -13192.782 with an uncertainty of ±0.047.
The mass was measured using Nuclear Physics and the related equations include:
25Mg has 12 protons and 13 neutrons.
25Mg is a stable isotope.
The mass excess of 25Mg is -13192.782 with an uncertainty of ±0.047.
The mass was measured using Nuclear Physics and the related equations include:25Mg has 12 protons and 13 neutrons. 25Mg is a stable isotope. The mass excess of 25Mg is -13192.782 with an uncertainty of ±0.047. The mass was measured using Nuclear Physics and the related equations include: