41K-Potassium
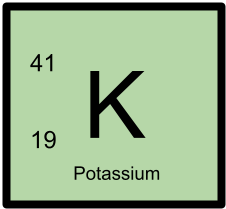 41K has 19 protons and 22 neutrons.
41K is a stable isotope.
The mass excess of 41K is -35559.5488 with an uncertainty of ±0.00376.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:
41K has 19 protons and 22 neutrons.
41K is a stable isotope.
The mass excess of 41K is -35559.5488 with an uncertainty of ±0.00376.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:
Influence Equations
99.9% 41K-40ArH 1
0.1% 40K(n, γ)41K 2
Reference
1. Brianna J. Mount, Matthew Redshaw, and Edmund G. Myers (2010) Atomic masses of 6Li, 23Na, 39,41K, 85,87Rb, and 133Cs
Physical Review A 82(4)
https://journals.aps.org/pra/abstract/10.1103/PhysRevA.82.042513
2. B. Krusche, K.P. Lieb, L. Ziegler, H. Daniel, T. von Egidy, R. Rascher, H.G. Borner, G. Barreau, D.D. Warner (1984)
Spectroscopy of 41K by thermal neutron capture in 40K Nuclear Physics A 417(2)
https://doi.org/10.1016/0375-9474(84)90506-2
 41K has 19 protons and 22 neutrons.
41K is a stable isotope.
The mass excess of 41K is -35559.5488 with an uncertainty of ±0.00376.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:
41K has 19 protons and 22 neutrons.
41K is a stable isotope.
The mass excess of 41K is -35559.5488 with an uncertainty of ±0.00376.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:41K has 19 protons and 22 neutrons. 41K is a stable isotope. The mass excess of 41K is -35559.5488 with an uncertainty of ±0.00376. The mass was measured using a Penning Trap and Nuclear Physics and the related equations include: