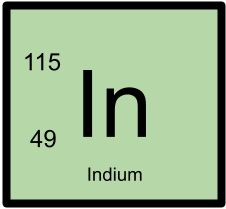
 115In has 49 protons and 66 neutrons.
115In is a stable isotope.
The mass excess of 115In is -89536.357 with an uncertainty of ±0.012.
The mass was measured using a Penning Trap and the related equations include:
115In has 49 protons and 66 neutrons.
115In is a stable isotope.
The mass excess of 115In is -89536.357 with an uncertainty of ±0.012.
The mass was measured using a Penning Trap and the related equations include:115In has 49 protons and 66 neutrons. 115In is a stable isotope. The mass excess of 115In is -89536.357 with an uncertainty of ±0.012. The mass was measured using a Penning Trap and the related equations include:
Influence Equations
100% 115In-129Xe 1
Reference
1. B.J. Mount, M. Redshaw, E.G. Myers (2009) Q Value of 115In → 115Sn(3/2+): The Lowest Known Energy β Decay
Physical Review Letters 103(12)
https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.103.122502