202Hg-Mercury
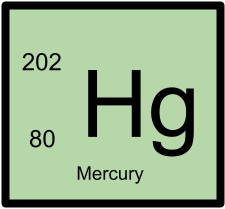 202Hg has 80 protons and 122 neutrons.
202Hg is a stable isotope.
The mass excess of 202Hg is -27345.31 with an uncertainty of ±0.705.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:
202Hg has 80 protons and 122 neutrons.
202Hg is a stable isotope.
The mass excess of 202Hg is -27345.31 with an uncertainty of ±0.705.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:
Influence Equations
37.5% 201Hg(n, γ)202Hg 1
28.3% 202Hg35Cl-200Hg37Cl 2
25.8% 204Hg35Cl-202Hg37Cl 2
Reference
1. D. Breitig, R.F. Casten, W.R. Kane, G.W. Cole, J.A. Cizewski (1975) Level structure and γ transitions in 202Hg studied by the (n, γ) reaction
Physical Review C 11(2)
https://journals.aps.org/prc/abstract/10.1103/PhysRevC.11.546
2. W. McLatchie, R.C. Barber, R.L. Bishop, H.E. Duckworth, B.G. Hogg, J.D. Macdougall, P.Van Rookhuyzen (1964) Some new neutron separation energies for isotopes of Hg, Tl, Pb, and Bi
Canadian Journal of Physics 42(5)
https://cdnsciencepub.com/doi/abs/10.1139/p64-086
 202Hg has 80 protons and 122 neutrons.
202Hg is a stable isotope.
The mass excess of 202Hg is -27345.31 with an uncertainty of ±0.705.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:
202Hg has 80 protons and 122 neutrons.
202Hg is a stable isotope.
The mass excess of 202Hg is -27345.31 with an uncertainty of ±0.705.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:202Hg has 80 protons and 122 neutrons. 202Hg is a stable isotope. The mass excess of 202Hg is -27345.31 with an uncertainty of ±0.705. The mass was measured using a Penning Trap and Nuclear Physics and the related equations include: