2H-Hydrogen 2
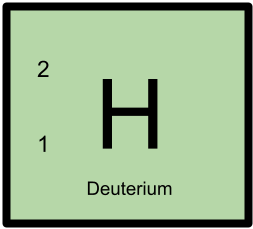 2H has 1 proton and 1 neutron.
2H is a stable isotope.
The mass excess of 2H is 13135.7229 with an uncertainty of ±0.000015.
The mass was measured using a Penning Trap and the related equations include:
2H has 1 proton and 1 neutron.
2H is a stable isotope.
The mass excess of 2H is 13135.7229 with an uncertainty of ±0.000015.
The mass was measured using a Penning Trap and the related equations include:
Influence Equations
82.5% D6-C 1
8.5% H2-D 2
8.2% HD-C 3
Reference
1. Conference on Mass Spectroscopy
C.M. Stevens, P.E. Moreland
2. Moreland, P.E., Bainbridge, K.T. (1964).
Direct Determination of the 3H—3He Mass Difference. In: Johnson, W.H. (eds) Nuclidic Masses.
Springer, Vienna.
https://link.springer.com/chapter/10.1007/978-3-7091-5556-1_37
3. Rau, S., Heiße, F., Köhler-Langes, F. et al. (2020), Penning trap mass measurements of the deuteron and the HD+ molecular ion. Nature
585(43-47)
https://www.nature.com/articles/s41586-020-2628-7?proof=t#citeas
 2H has 1 proton and 1 neutron.
2H is a stable isotope.
The mass excess of 2H is 13135.7229 with an uncertainty of ±0.000015.
The mass was measured using a Penning Trap and the related equations include:
2H has 1 proton and 1 neutron.
2H is a stable isotope.
The mass excess of 2H is 13135.7229 with an uncertainty of ±0.000015.
The mass was measured using a Penning Trap and the related equations include:2H has 1 proton and 1 neutron. 2H is a stable isotope. The mass excess of 2H is 13135.7229 with an uncertainty of ±0.000015. The mass was measured using a Penning Trap and the related equations include: