58Fe-Iron
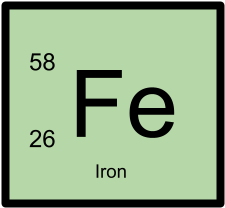 58Fe has 26 protons and 32 neutrons.
58Fe is a stable isotope.
The mass excess of 58Fe is -62155.271 with an uncertainty of ±0.316.
The mass was measured using Nuclear Physics and the related equations include:
58Fe has 26 protons and 32 neutrons.
58Fe is a stable isotope.
The mass excess of 58Fe is -62155.271 with an uncertainty of ±0.316.
The mass was measured using Nuclear Physics and the related equations include:
Influence Equations
86.4% 57Fe(n, γ)58Fe 1
9.4% 58Fe(n, γ)59Fe 2
4.2% 58Fe(p, γ)59Co-56Fe()57Co 3
Reference
1. M.A. Islam, T.J. Kennett, S.A. Kerr, W.V. Prestwich (1980) A self-consistent set of neutron separation energies
Canadian Journal of Physics
https://cdnsciencepub.com/doi/abs/10.1139/p80-028
2. A.M.J. Spits, J.A. Akkermans (1973) Investigation of the reaction 37Cl(n, γ)38Cl
Nuclear Physics A 215(2)
https://www.sciencedirect.com/science/article/pii/0375947473906556#!
3. A. Brondi, R. Moro, P. Pelter, F. Terassi (1975) Gamma-decay of the fragmented analogue of the 59Fe ground state
Nuovo Cimento A (1965-1970) 30(483-497)
https://link.springer.com/article/10.1007/BF02730298
 58Fe has 26 protons and 32 neutrons.
58Fe is a stable isotope.
The mass excess of 58Fe is -62155.271 with an uncertainty of ±0.316.
The mass was measured using Nuclear Physics and the related equations include:
58Fe has 26 protons and 32 neutrons.
58Fe is a stable isotope.
The mass excess of 58Fe is -62155.271 with an uncertainty of ±0.316.
The mass was measured using Nuclear Physics and the related equations include:58Fe has 26 protons and 32 neutrons. 58Fe is a stable isotope. The mass excess of 58Fe is -62155.271 with an uncertainty of ±0.316. The mass was measured using Nuclear Physics and the related equations include: