112Cd-Cadmium
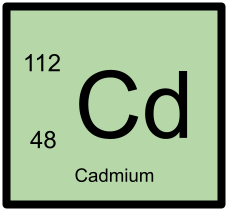 112Cd has 48 protons and 64 neutrons.
112Cd is a stable isotope.
The mass excess of 112Cd is -90574.857 with an uncertainty of ±0.25.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:
112Cd has 48 protons and 64 neutrons.
112Cd is a stable isotope.
The mass excess of 112Cd is -90574.857 with an uncertainty of ±0.25.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:
Influence Equations
48.4% 113In-112Cd1.009 1
35.2% 113Cd-112Cd1.009 1
8.3% 111Cd(n, γ)112Cd 2
Reference
1. N.D. Gamage, G. Bollen, M. Eibach, K. Gulyuz, C. Izzo, R.M.E.B. Kandegedara, M. Redshaw, R. Ringle, R. Sandler, A.A. Valverde (2016) Precise determination of the 113Cd
fourth-forbidden of non-unique β-decay Q value Physical Review C 94(2)
https://journals.aps.org/prc/abstract/10.1103/PhysRevC.94.025505
2. H.E. Jackson, L.M. Bollinger (1961) Isotopic Identification of Neutron Resonances of Cd, Sb, Os, and Pt from Capture Gamma-Ray Spectra
Physical Review 124(4)
https://journals.aps.org/pr/abstract/10.1103/PhysRev.124.1142
 112Cd has 48 protons and 64 neutrons.
112Cd is a stable isotope.
The mass excess of 112Cd is -90574.857 with an uncertainty of ±0.25.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:
112Cd has 48 protons and 64 neutrons.
112Cd is a stable isotope.
The mass excess of 112Cd is -90574.857 with an uncertainty of ±0.25.
The mass was measured using a Penning Trap and Nuclear Physics and the related equations include:112Cd has 48 protons and 64 neutrons. 112Cd is a stable isotope. The mass excess of 112Cd is -90574.857 with an uncertainty of ±0.25. The mass was measured using a Penning Trap and Nuclear Physics and the related equations include: