13C-Carbon
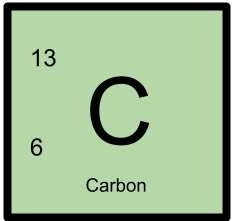 13C has 6 protons and 7 neutrons.
13C is a stable isotope.
The mass excess of 13C is 3125.00933 with an uncertainty of ±0.00023.
The mass was measured using a Penning Trap and the related equations include:
13C has 6 protons and 7 neutrons.
13C is a stable isotope.
The mass excess of 13C is 3125.00933 with an uncertainty of ±0.00023.
The mass was measured using a Penning Trap and the related equations include:
Influence Equations
78.2% 13CH-14N 1
21.8% 13CH2-28Si 2
0.2% 136Xe-13C3O6 3
Reference
1. Simon Rainville, James K. Thompson, David E. Pritchard (2004), An Ion Balance for Ultra-High-Precision Atomic Mass Measurements Science
303(5656)
https://www.science.org/doi/10.1126/science.1092320
2. Matthew Redshaw, Joseph McDaniel, and Edmund G. Myers (2008), Dipole Moment of PH+ and the Atomic Masses of 28Si,31P by Comparing Cyclotron Frequencies of Two Ions Simultaneously Trapped in a Penning Trap
Physical Review Letters, 100(9)
https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.100.093002
3. Matthew Redshaw, E. Wingfield, Joseph McDaniel, and Edmund G. Myers (2007), Mass and Double-Beta-Decay Q Value of 136Xe
Physical Review Letters, 98(5)
https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.98.053003
 13C has 6 protons and 7 neutrons.
13C is a stable isotope.
The mass excess of 13C is 3125.00933 with an uncertainty of ±0.00023.
The mass was measured using a Penning Trap and the related equations include:
13C has 6 protons and 7 neutrons.
13C is a stable isotope.
The mass excess of 13C is 3125.00933 with an uncertainty of ±0.00023.
The mass was measured using a Penning Trap and the related equations include:13C has 6 protons and 7 neutrons. 13C is a stable isotope. The mass excess of 13C is 3125.00933 with an uncertainty of ±0.00023. The mass was measured using a Penning Trap and the related equations include: