Example 7.4 Using Henry’s
Law
Estimate the molar solubility (the solubility in moles per liter)
of oxygen in water at 25 0C and a partial pressure of 160 torr (its partial pressure
in the atmosphere at sea level). The
Henry’s constant for O2 in water at 298 K is 3.30 x 107
torr.
Method The mole fraction
is given by Henry’s law as
xO = pO/K, where pO is the
partial pressure of oxygen. Use mole
fraction to solve for moles of O2.
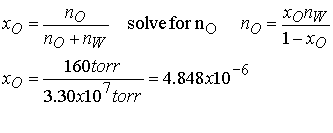
Since the mole fraction of O2 is so low we can use the
moles of pure water for the moles of water in the solution.
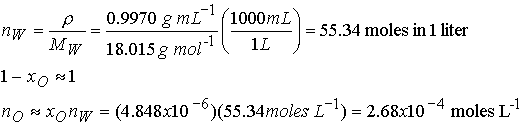
Divers have to be concerned about the solubility of O2
and N2 in blood since the concentration of dissolved gases in blood
increases as pressure is increased. If
a diver comes to the surface too quickly (where the solubility of N2
is lower) the N2 can form bubbles of gas in blood veins and
capillaries and cause the pain of the bends.
Deep sea divers breathe a mixture of 98% He, 2% O2. He has a much lower solubility in blood
than N2. At a pressure of 10
atm this gives an O2 partial pressure of about 0.2 atm (the pressure at atmosphere).
The properties of solutions
7.4 Liquid mixtures
Gibbs energy of mixing of two liquids calculated in the same way as for gases (7.2a)
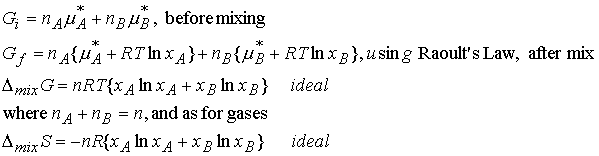
For perfect gases there are no interactions between the
molecules. For ideal solutions there
are interactions, however the A-A , A-B, and B-B interactions are all
equal. The enthalpy of mixing is zero.
Real gases have different A-A, A-B, and B-B interactions. There may be an enthalpy change and
possibly an entropy change when the liquids mix. Gibbs free energy can be positive or negative. In the case of DG > 0, the components
spontaneously separate (immiscible).
In some cases, the two liquids may be soluble over a limited range (partial
miscibility).
![]()
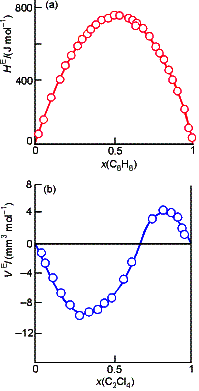
The
thermodynamics of real solutions may be expressed in terms of excess
functions, XE, the difference between the observed
thermodynamic function of mixing and the function of an ideal solution. For example, the excess entropy:
where DmixSideal was given
earlier. The excess enthalpy, HE,
and volume, VE, are both equal to the observed enthalpy and volume
of mixing, because the ideal values are zero in each case.
Deviations of the excess energies from zero indicate the extent to
which solutions are non-ideal. We
define a regular solution model.
One in which the two types of molecules distribute randomly (SE = 0), but have
different energies of interaction (HE not equal to 0).
7.5 Colligative properties
•Boiling point elevation
•freezing point depression
•osmotic pressure
Arise from the presence of solute particles independent of
identity. Properties are proportional
to the number of particles. The following expressions are derived with two
assumptions:
1.
solute is non-volatile ( has no vapor pressure in the
solution)
2.
the solid solvent and
solute are immiscible (freezing pt. Depression)
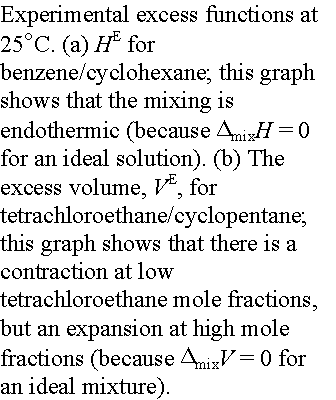
(a) The common features of colligative properties
All the colligative properties stem from the reduction of the chemical potential of the liquid solvent as a result of the presence of solute.

Molecular interpretation 7.3
The molecular origin of the lowering of chemical potential is not
the energy of interaction of the solute and solvent molecules, because lowering
occurs in ideal solutions (with zero enthalpy of mixing). It must be an entropy effect.
Boiling point elevation.
The pure liquid solvent has an entropy which reflects the disorder of
its molecules. Its vapor pressure
reflects the tendency of solution towards greater entropy, which is achieved by
vaporization into the more highly disordered gas phase. When a solute is present, there is an
additional contribution to the entropy of the fluid (even in an ideal solution). The entropy of the solution is higher
than that of the pure solvent and thus there is a weaker tendency to form the
gas. The effect of solute appears
as a lower vapor pressure and hence a higher boiling point.
Freezing point depression. Similarly, the enhanced molecular randomness
of the solution opposes the tendency to freeze. Consequently, a lower temperature must be reached before
equilibrium between solid and solution is achieved. Thus, the freezing point is lowered.
Strategy for quantitative discussion of boiling point elevation
and freezing point depression is to find the temperature at which, one phase
(pure solvent vapor or pure solid solvent) has the same chemical potential
as the solvent in solution. This
will be the new equilibrium temperature.
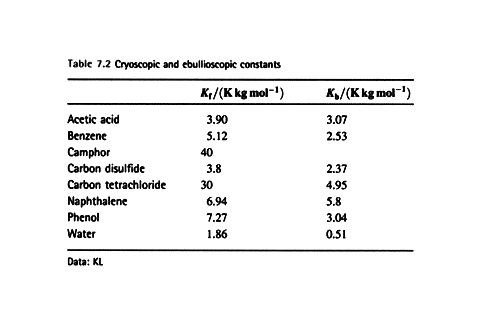
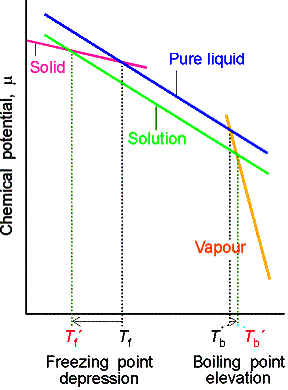
(b) The elevation of boiling point
We again denote the solvent by A and the solute by B for this
heterogeneous equilibrium.
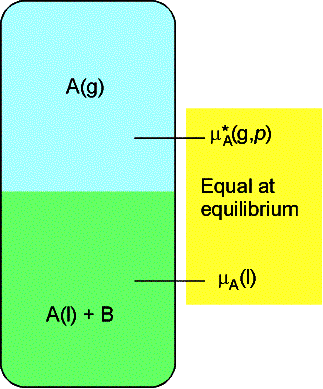
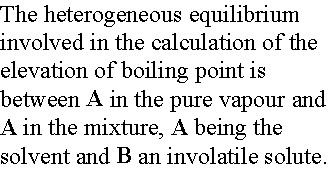
The equilibrium is established at a temperature for which
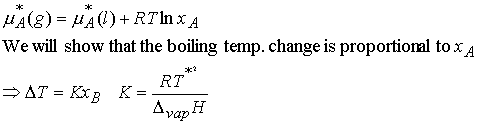
where T* is the normal boiling point and DT is the boiling
point elevation.
Justification 7.2
First substitute 1 – xB = xA and rearrange the equilibrium chemical potential expression.
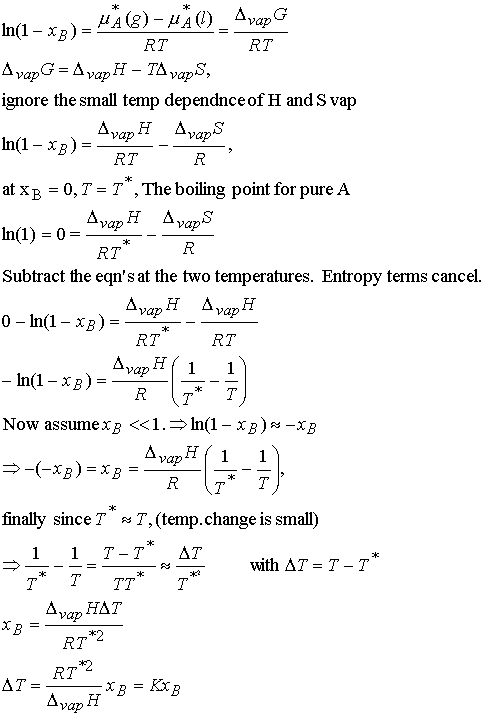
The value of DT does depend upon the properties of the
solvent and not the solute. The biggest
change occurs for solvents with high boiling points. For practical applications we note that the mole fraction of B is
proportional to the molality, b, in dilute solution.
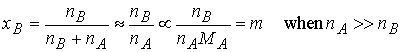
and write
![]()
where Kb is the empirical ebullioscopic
constant of the solvent.