By the same argument that led to the total volume as a function of
composition we can write
![]()
In an open system of constant composition, the Gibbs energy
depends upon p, T, and composition(nJ). Thus the
equation dG = Vdp -SdT becomes

That is, non-expansion work can arise from the changing composition of a
system. (electrical cell)
(c) The wider significance off chemical potential

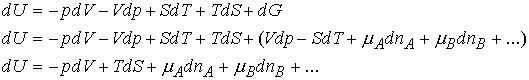
This expression is the generalization of
dU = TdS – pdV to include
multiple components.
It follows that at
constant volume and entropy,
![]()

Similarly,

Thus mJ shows how all the extensive
thermodynamic properties U, H, A, and G, depend on the composition.
The Gibbs-Duhem equation
Because the total Gibbs energy of a mixture is given by
![]()
and the chemical potentials depend on the composition, when
compositions are changed infinitesimally we write
![]()
At constant temperature and pressure,
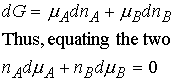
or more generally, the Gibbs-Duhem equation
![]()
The chemical potential of one component of a mixture cannot change
independently of the other. In a binary
mixture, if one partial molar quantity increases the other must decrease.

We can derive a similar equation for any other partial molar
quantity. For example, the partial
molar volume…

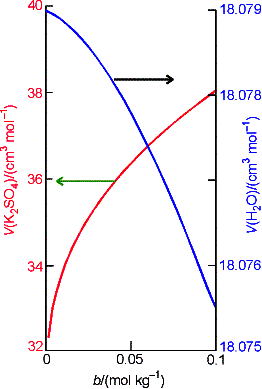
Example 7.1 Using the Gibbs-Duhem Equation
The experimental value of the partial molar volume of K2SO4(aq) at 298 K is
given by the expression
![]()
where b is the numerical value of molality of K2SO4. Use the Gibbs-Duhem equation to derive an
equation for the molar volume of water in the solution. The molar volume of pure water at 298 K is
18.079 cm3 mol-1.
Let A denote K2SO4 and W denote
water.
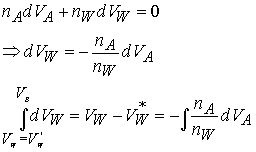
Next, change the variable VA to the molality
b. Take the derivative of VA
to get the differential of b. Then integrate the right hand side from
b = 0 (pure water) to b.
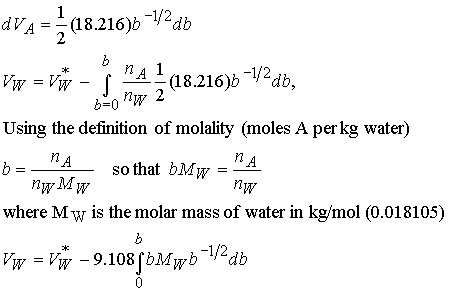

We
have derived an expression for the molar volume of water (as a function of
molality) from the molar volume of K2SO4 using their
relationship in the Gibbs-Duhem equation.