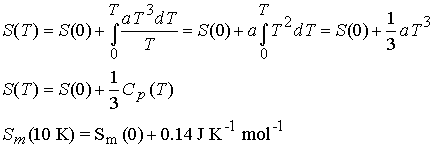The Clausius inequality.
So far we have proven S is a state function.† We now need to verify that entropy is a
signpost for spontaneous change.
![]()
Consider a system in thermal and mechanical contact with its
surroundings at the same temperature, T (system & surroundings are not
necessarily in mechanical equilibrium). Any change of state is accompanied by a
change in entropy of the system, dSsys, and of the surroundings, dSsur.† Because the process might be irreversible,
the total entropy will increase when a process occurs in the system, we can
write
![]()
The equality applies if the process is reversible.
When dq is the heat supplied to the system by the surroundings (dqsur = -dqsys).
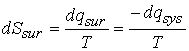
†It follows that for any
change,†
![]() †††††††† The Clausius
inequality
†††††††† The Clausius
inequality
If the system is isolated from its surroundings, then dqsys = 0, and† dSsys ≥ 0† This implies that the entropy in an isolated system can not
decrease when a spontaneous process takes place.†
Example 1-† A system undergoes
irreversible adiabatic change:
dq = 0,†††† ![]() †and dSsur = 0, & dStot ≥ 0.
†and dSsur = 0, & dStot ≥ 0.
Example 2. - A perfect gas undergoes irreversible isothermal expansion
freely into a vacuum.†† From ex.
4.1,† dq = -dw, (because dU = 0).† dw = 0 (vacuum), implies dq = 0, too.† According to Clausius inequality, dSsys† ≥ 0.† For the surroundings, no heat is
transferred, so dSsur = 0, and in this case dStot ≥ 0 also.††
Example 3†† Consider the
irreversible process of spontaneous cooling.†
Entropy of hot source changes by†
-ĹdqĹ/Th (a
decrease).†
Entropy of the cold sink changes by† +ĹdqĹ/Tc.†
Overall entropy change is
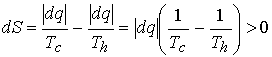
which is positive since, Th ≥ Tc.† Hence cooling, the transfer of heat, is spontaneous.††
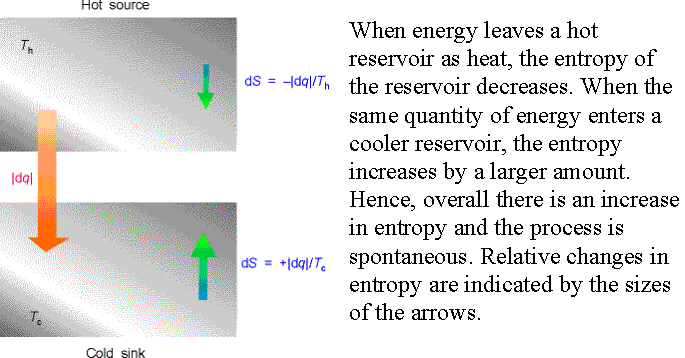
Entropy
change is proportional to temperature.†
For the same heat transfer, dq, entropy change is larger for the lower
temperature.† (A more pronounced effect
at lower temperature the system is more organized and has fewer modes of heat
dissipation at lower temperature.
Entropy changes accompanying specific processes.
a) Entropy of phase transition at transition temperature
For liquid vaporization, the substance goes from a more compact
dense phase with more order to a widely dispersed gas.† We expect an increase in entropy to
accompany this transition.
Consider an equilibrium mixture of water and ice at 273 K,
(the normal transition temperature Ttrs,) and 1atm.† At the transition temperature, any transfer
of heat between the system and surroundings is reversible because
the two phases in the system are in equilibrium.† At constant pressure,† q =
DtrsHm
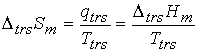
If the phase transition is exothermic (DtrsH < 0, as in
freezing or condensing), then entropy is negative.† The system has become more ordered.† If the transition is endothermic, enthalpy positive, the entropy
change is positive.† This is consistent
with the system becoming more disordered.†
The entropy of system can be negative as long as DSsur
is equal or larger and positive so that DStot
obeys the Clausisu inequality.
Some experimental entropies of transition.
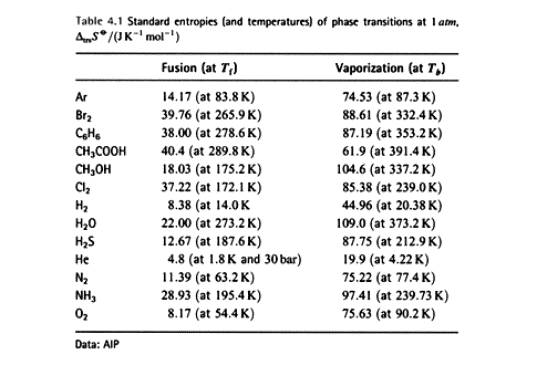
Interesting feature: a large number of fluids give ~ the same standard
entropy of vaporization ( about 85 J K-1)†
- Troutonís Rule †(a rule
of thumb)††††† 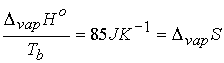
Molecular interpretation of Troutonís rule.
A comparable amount of disorder is generated when any liquid
evaporates and becomes a gas.† (most
liquids similar)
Some do not follow the rule (such as water and alcohols) which are
more ordered in the liquid due to hydrogen bonding.†
Example 4.2 Using Troutonís rule
Predict the standard molar enthalpy of vaporization of
bromine†† using Troutonís rule given
that it boils at 59.2 0C.
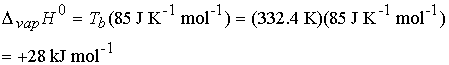
The experimental value is +29.45 kJ mol-1.†
b) The expansion of a perfect gas.† Isothermal expansion
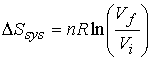
Since S is a state function, the expression above applies
whether the transition is reversible or irreversible.†
†††††††
For reversible change †† ††DStot = 0, therefore DStot = -DSsys
For irreversible change such as free expansion w = 0 and if †††††† T remains constant, then q = 0.† Therefore, DSsur = 0
††††††† and DStot =† DSsys.††
c). The variation of entropy with temperature
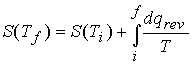
![]()
At constant pressure, with no expansion work,
Consequently,†††††††† 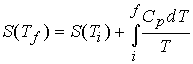
At constant volume
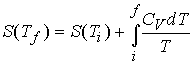
If Cp can be
considered constant
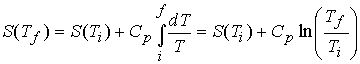
Example†† Calculate the
entropy change when argon at 25 0 C and 1.00 atm in a container of
volume 500 cm3 is compressed to 50.0 cm3 and is
simultaneously cooled to -250 C.†
Method:† Since S is a state function we can choose
any convenient reversible path.† We will break the process into two
reversible steps.† First step is
reversible isothermal compression to the final volume followed by the second
step, reversible cooling at constant volume to the final temperature.
We need to compute n from the ideal gas law.† †Use 1 L = 1 dm3†††
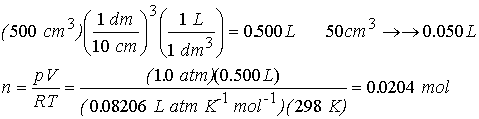
Step
1† reversible, isothermal compression
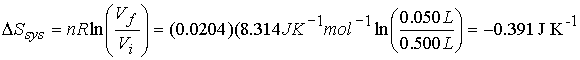
Step
2† reversible cooling at constant volume
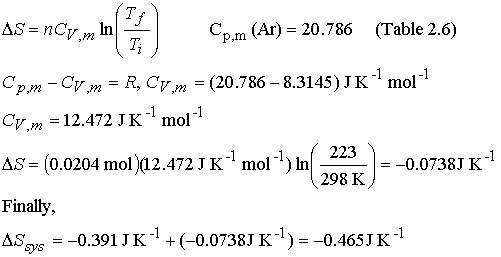
The
entropy of the system decreases as it is cooled and compressed, both increase
the order of the molecules, fewer molecular states are available.
Measurement of entropy
The entropy of a system at temperature T is related to its entropy
at T = 0 by measuring its heat capacity, Cp, at different
temperature ranges and evaluating the following integral.
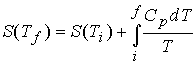
Entropy of transitions must be added (DtrsH/Ttrs), for example a
substance that melts (Tf ) and boils (Tb).†
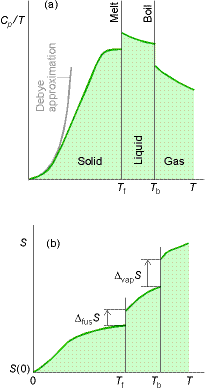
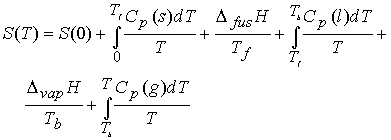

Heat capacities are difficult to measure near zero T. The Debye
Extrapolation indicates that heat capacities at low T follow the following
relationship†††† † ![]()
Cp is determined at as low a T as possible and the data is fit
to the function aT3 to determine a .
Metals at very low
temperature also have a contribution to the heat capacity from the
electrons.† At low enough temperature
the free flow of electrons (conduction) becomes restricted.† The molar heat capacity is linearly
proportional to temperature.
Example 4.4† Calculating the
entropy at low temperatures.†
The molar constant-pressure heat capacity of a certain solid at 10
K is 0.43 J K-1 mol-1.† What is its molar
entropy at that temperature?†††