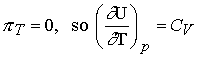Chapter 3 First Law: The Machinery
The Mathematics of Thermodynamics:
The power of thermodynamics is in being able to relate measurable
properties to those that are not so easily measured. Many of the relationships involve the slopes of functions, the
rate of change of one variable with respect to another, the derivative. To understand how these relationships are
derived we need to understand some mathematical properties of differentials and
partial derivatives. See Further
Information 1, pg. 905-906.
Exact differentials
Recall that change in internal energy, dU, is called an exact differential because it depends only on initial and final state of the system but not the path. We will define exact differential mathematically.
If we have a function arbitrarily designated by J which depends upon the variables T and p so that we may write J = J(T, p), then the infinitesimal change in J, dJ, is given by
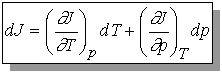
The variables T and p must be independent and J(T,p) must be
continuous, single-valued, differentiable.
(no step functions, one value of J for every T and p, no functions
with cusps)
The partial derivatives will, in general, also be functions of T
and p, derivable from J(T,p) by standard differentiation techniques. We could
therefore define the functions M and N by
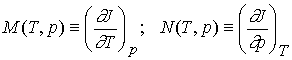
The expression for dJ becomes
dJ = MdT + Ndp
We
know that the order of differentiation doesn’t matter for second
derivatives.
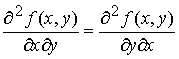
If the original function J(T,p) is continuous, single-valued and
differentiable, then
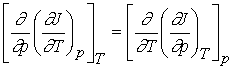
Inserting
M and N for their differentials
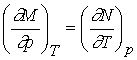
This condition is sufficient as well as necessary for an exact
differential. That is, given an
expression in differential form P(Q,S) and R(Q,S),
if 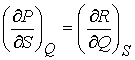 then there exists
a function f(Q, S) such
then there exists
a function f(Q, S) such
that df = PdQ + RdS.
If these conditions are met, then df is called an exact
differential.
If the function f(Q, S) is single-valued; that is, if for each set
of values of Q and S one and only one value of f exists, then changes in f as Q
and S change depend only upon the initial and final values of Q and S. That is, we may write
![]()
in which the last expression means “Df is a function
only of Q1, Q2, S1 and S2”.
Line integrals
Another way we could evaluate changes in the function f is to
integrate the differential. We may
write
![]()
Since the differential df is exact, this integral will depend only
upon S1, Q1, S2, and Q2. There are an
infinite number of ways we can go from S1, Q1 to S2, Q2 since these are
completely independent variables. For
example we may hold S constant at S1 while changing Q from Q1 to Q2, and the hold Q
constant at Q2 while varying S from S1 to S2.
![]()
This is called a path. The integral of an infinitesmal quantity along such a path is called a line integral. The form of the integrals will be different for different paths but the line integral of an exact differential depends only on the initial and final values of the independent variables and not upon the path of integration.
Let’s
test a differential for exactness.
Example 1
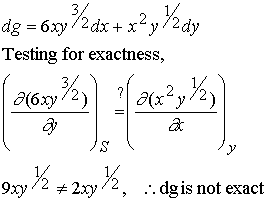
Example 2: Show that the
differential dVm of the molar volume of an ideal gas is an exact
differential.
First write the differential expression.
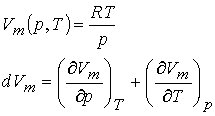
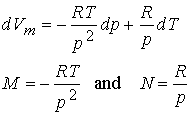
Then take the first
partial derivatives
Finally
take the second partial derivatives
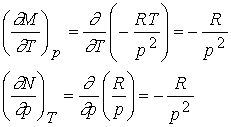
They
are equal so Vm is an exact differential. Therefore Vm is a state function and depends only on
initial and final state.
We
will use some other relationships (identities) for partial derivatives (pg.
906).
State functions and exact differentials

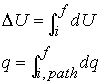 dU depends only on
the initial and final states. dU is exact. q is the
sum of the individual contributions of
heat transferred at each point along a path. dq is inexact.
dU depends only on
the initial and final states. dU is exact. q is the
sum of the individual contributions of
heat transferred at each point along a path. dq is inexact.
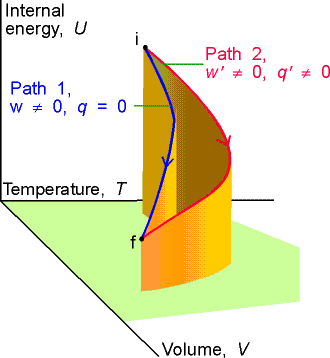
Calculating work, heat, and internal energy
Consider a perfect gas inside a cylinder fitted with a
piston. Let the initial state be T, Vi and the final
state be T, Vf. The change of state can be brought about in many ways, two
of which are: Path 1, in which there is irreversible isothermal expansion
against a constant pressure; Path 2, in which there is reversible, isothermal
expansion. Compute w, q, and DU for each path.
DU = q + w
DU = 0
for any isothermal change of a perfect gas.
q = -w
Path 1. irreversible
w = -pexdV
so q = + pexdV
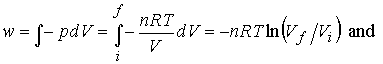
Path
2. reversible
![]()
We see that DU is the same for each path but q and w
are different by paths 1 and 2.
Changes in internal energy
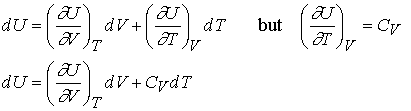
For a
closed system, constant n, U is a function of volume and temperature. Now suppose that V and T both change
infinitesimally
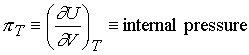
Internal
pressure is a measure of the strength of the cohesive forces of the sample (intermolecular
forces)
![]()
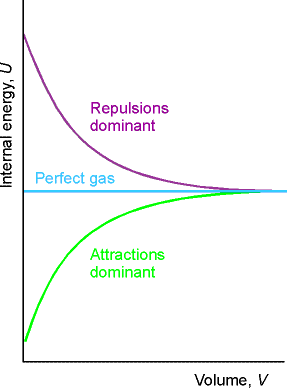

Illustration
For ammonia, pT = 840 Pa at 300
K and 1.0 bar, and CV,m = 27.32 J K-1 mol-1. The change in molar internal energy of
ammonia when it is heated through 2.0 K and compressed through 100 cm3 is ~
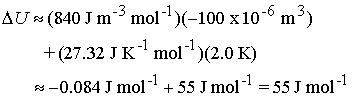
Note
that the heating term dominates the internal energy
Changes in internal energy at constant pressure
How does internal energy vary with temperature when the pressure
of the system is constant.
![]()
Divide both sides by dT and impose constant pressure gives
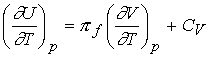
Defining the expansion coefficient, a 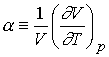
gives 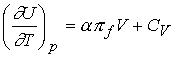
A large value for a means the volume responds strongly to
changes in temperature.
Example - using the expansion coefficient of a gas
Derive an expression for the expansion coefficient of a perfect
gas.
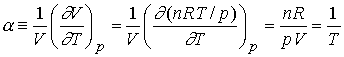
The
internal pressure for a perfect gas is zero, non-interacting.