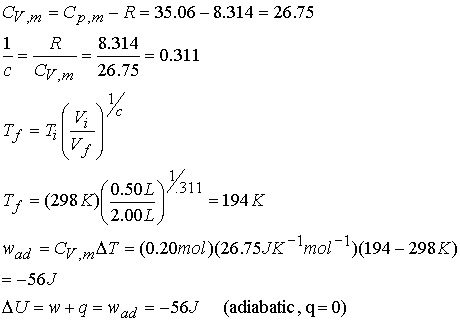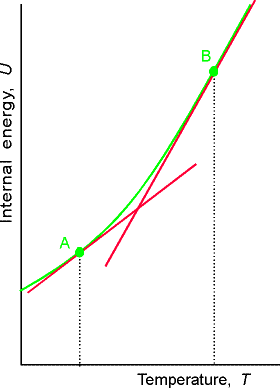

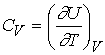
Over
small temperature ranges the slope changes very little – Cv may be
considered a constant.
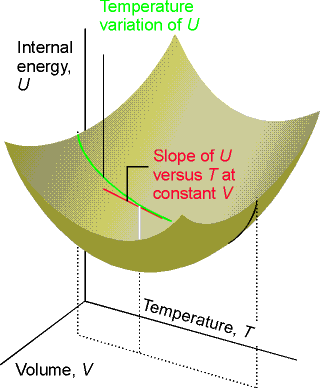
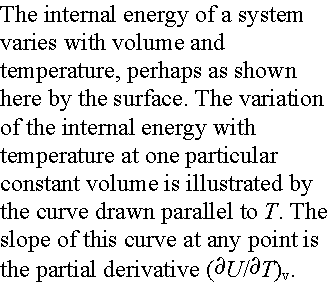
Heat capacity is an extensive property. Molar heat capacity, Cv,m is an intensive
property. (units J K-1 mol-1)
Specific heat capacity is the heat capacity divided by
mass. (units J K-1 g-1
= J °C-1 g-1)
In general, heat capacities depend on temperature and decrease at
low temperatures.
The heat capacity for a monatomic perfect gas is:
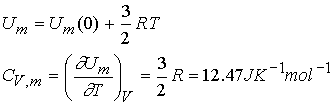
It follows that: dU = CVdT and
DU =CVDT = qV
Enthalpy: The change in internal energy is not equal to the heat
supplied when the system is free to change its volume. Some energy is returned to the surroundings as
expansion work so
dU < dq.
H = U + PV
P and V are the pressure and volume of the system. Enthalpy is a convenient state function for systems under constant pressure.
The change in Enthalpy is equal to the heat supplied to the system
(so long as the system does no additional work).
dH = dq (at
constant pressure, no additional work)
DH = qp macroscopic measurement
Justification
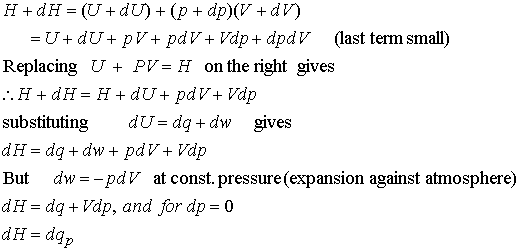
Measurement of enthalpy change: Calorimetry - measuring
temperature change accompanying a chemical reaction at constant pressure. Another route is to use bomb calorimetry for
solids and liquids where pV is small and dU » dH.
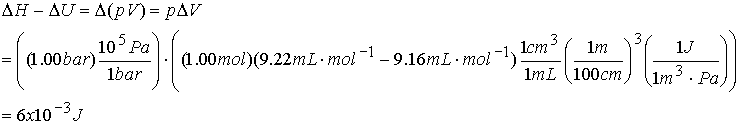
Example of relating DH and DU:
Calculate DH - DU for heating 1.00 moles of
zinc(s) from 25 °C to 98 °C at 1.00
bar. The Zn undergoes a volume change
from 9.16 mL mol-1 to 9.22 mL mol-1.
DU is typically a
value in kJ so pressure/volume work (pV) is negligible.
For a perfect gas
undergoing the same T increase,
the result is = 607 J
For a perfect gas:
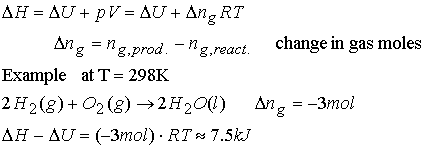
Example: Calculating a change in enthalpy
Water is heated to boiling under pressure of 1.0 atm. When an electric current of 0.50 A from a 12 Volt supply is passed for 300 seconds through a resistance in thermal contact with the water, it is found that 0.798 g of water is evaporated. Calculate molar internal and enthalpy changes at the boiling point (373.15 K).
Constant pressure:
Enthalpy = heat supplied
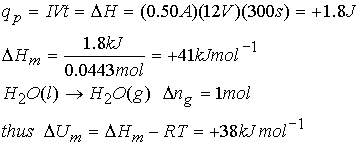
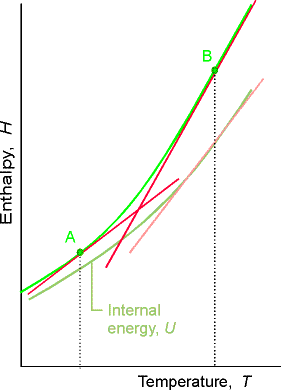
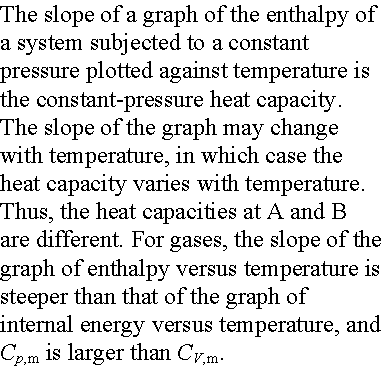
Variation of enthalpy with temperature:
Enthalpy increases with temperature at constant pressure. The heat capacity at constant pressure
is defined as:

![]()
Cp is a function of temperature and can be approximated by the
following expression
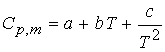
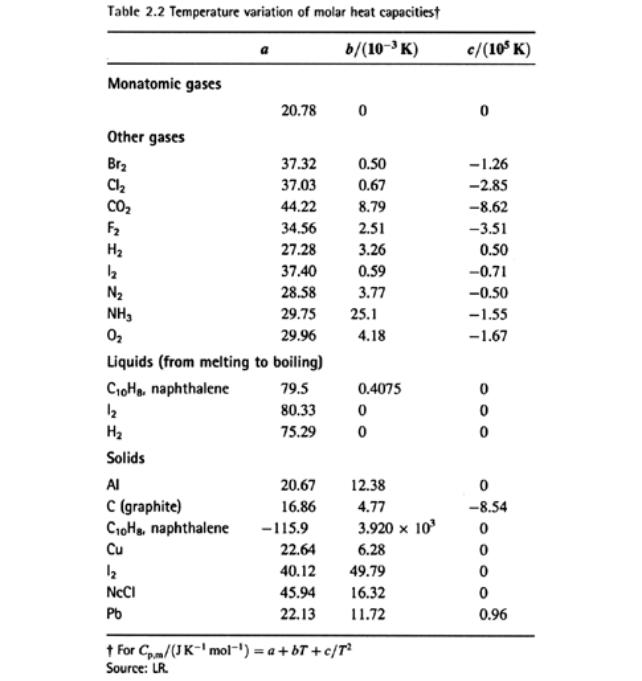
Example: What is the change
in molar enthalpy of carbon dioxide when heated from 25 °C to 100 °C ?
For CO2 a =
44.22, b = 8.79 x 10-3, c =
-8.62 x 105
Cp,m = a + bT + cT-2
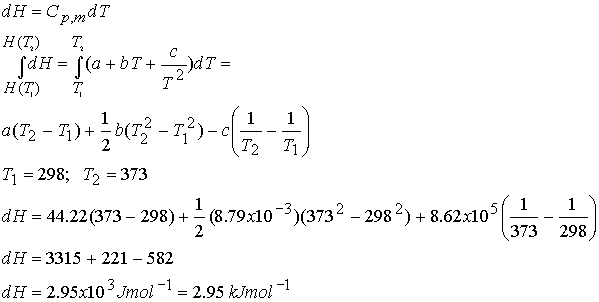
Relationship between heat capacities:
The constant pressure heat capacity (Cp) of a sample is greater than the constant volume heat
capacity because in the former case the energy supplied as heat is used to do
work of expansion, both against external pressure and (if there are
intermolecular forces) against the cohesive forces within the sample. For a perfect gas
![]()
will be derived later.
Cp,m=
Cv,m + R
For
a monatomic gas Cv,m = 3/2R
So
Cp,m = 3/2R + R = 5/2R
Adiabatic changes:
When a perfect gas expands adiabatically (q = 0)
we now expect T to decrease because:
•work is done
•the internal energy falls
•therefore the temperature of the working gas falls
The work of adiabatic change:
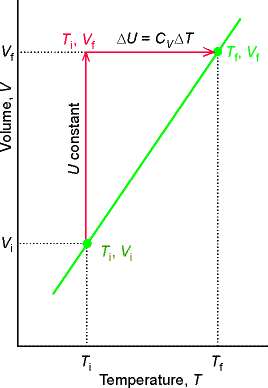
To achieve a change of state from one temperature and volume to another temperature and volume, we may consider the overall change as composed of two steps. In the first step, the system expands at constant temperature; there is no change in internal energy if the system consists of a perfect gas. In the second step, the temperature of the system is increased at constant volume. The overall change in internal energy is the sum of the changes for the two steps.
Because the internal energy of a perfect gas arises solely from
the kinetic energy of the molecules, overall change in internal energy arises
from the second step.
For
an adiabatic process, q=0 by definition.
DU = q + w = wad
DU = CVDT
(provided CV is independent of temp.)
wad
= CVDT
For the important case of an adiabatic reversible expansion relate the change in volume to the change in temperature.
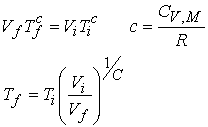
Use this expression to determine temperature change from the volume change.
Prove
the equation.

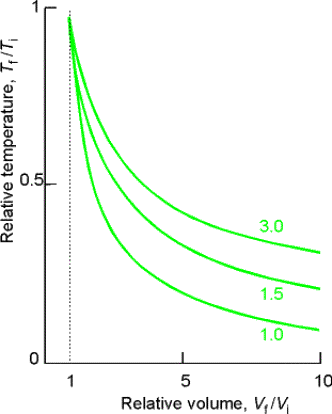
Example: Consider the adiabatic reversible expansion of 0.20 mol NH3,
initially at 25 0C, from 0.5 L to 2.00 L.
Cp,m for NH3 = 35.06 JK-1mol-1, so c =
1.501. Determine the final T, DU, and w.