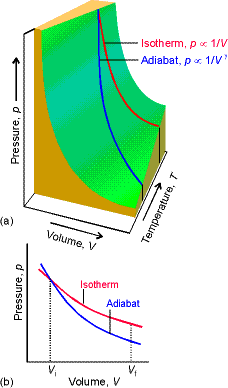
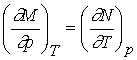Heat capacity ratios and adiabats:
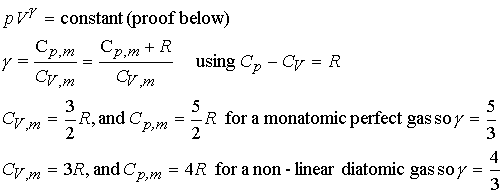
Consider Dp for an
adiabatic, reversible expansion of a perfect gas.
Proof

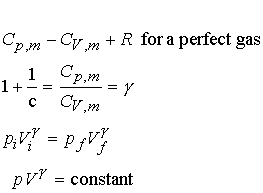

Thermochemistry:
The study of heat produced or required for chemical reactions.
Calorimetry is used to measure heat:
-at constant volume qV
= DU , internal
energy
-at constant pressure
qp = DH, enthalpy
*If we know DU or DH for a reaction, we can predict
the heat that will be produced.
Define: Standard enthalpy change = DH0, where initial and
final substances are in their standard state.
Standard state of a pure substance is 1 bar.
Example: standard enthalpy of vaporization
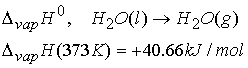
All kinds of enthalpies- Enthalpies of physical change
Standard enthalpy of transition = is the standard
enthalpy for a physical change.
![]()
Enthalpy is a state function which is independent of path. This is very significant in thermochemistry.
solid ® vapor
(sublimation)
solid ® liquid ® vapor Same enthalpy for both!
Also, as a state function,
![]()
Enthalpies of chemical change:
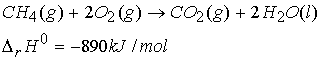
Standard
reaction enthalpy = DrH0
The combination of a chemical reaction and a standard reaction
enthalpy is called a thermochemical equation.
Consider 2A + B ® 3C + D

Example:
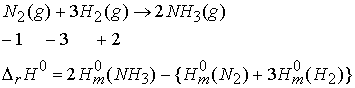
Hess’s Law: The standard enthalpy of an overall reaction is the sum of the standard enthalpies of the individual reactions into which a reaction may be divided.
The standard enthalpy of formation, DfH0, of a substance is
the reaction enthalpy for the formation of the compound from its elements in
their reference states.
The reference state of an element is its most stable state at
the specified temperature and 1 bar.
Example - benzene @ 298 K
![]()
![]()
The standard
enthalpies of formation for the elements in their reference state are 0 at all
temperatures. ie
Reaction enthalpy in terms of enthalpies of formation.
Conceptually regard a reaction as proceeding by decomposing the reactants into
their elements and then forming those elements into products. The value of DrH0 is the sum of
the forming and unforming enthalpies.
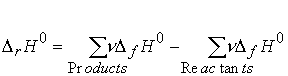
Illustration:
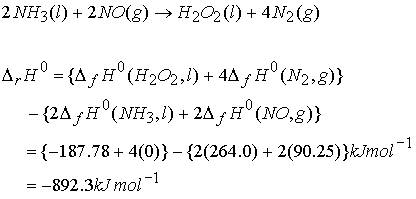
Formally,

Example
of Hess’s Law:

DrHo = -124 +(2220)
+ -(-286) = -2058 kJ mol-1
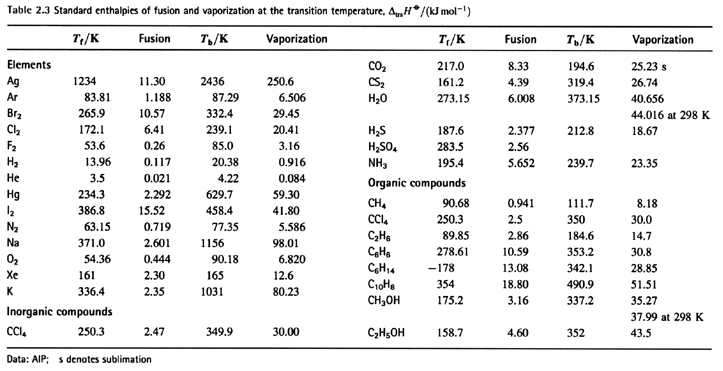
Thermochemical Group Contributions:
An approach to compute standard reaction enthalpies from knowledge
of the composition of the chemical species.
A molecule is
regarded as being built up of thermo-
chemical groups. The enthalpy of formation of the
the compound is then expressed
(approximately) as the
sum of the
contributions associated with all the
thermochemical groups
of the molecule.
Example: Thermochemical group approach.
Estimate of standard enthalpy of formation at 298 K of
2,2-dimethylpropane in a) the gas phase, b) the liquid phase.
First, identify the thermochemical groups present in the molecule.
There are four CH3(C) groups and one C(C)4 groups.

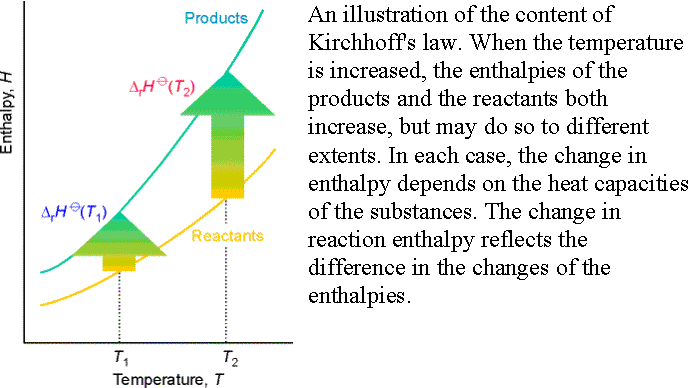
Temperature dependence of reaction enthalpies:
In the absence of standard enthalpy data for a substance at a
given T, it can be estimated from heat capacities and the reaction enthalpy at
some other temperature.
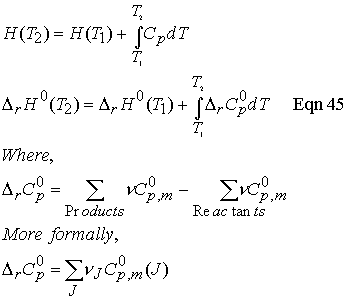
Equation 45
is known as Kirchhoff’s law and is normally a good approximation
to assume Dr Cp is independent
of temperature.
Example: The standard enthalpy of formation of gaseous H2O at 298 K is
-241.82 kJ mol-1. Estimate the value
at 100 0C given the following values of molar heat capacities at
constant pressure: H2O(g) : 33.58 kJ mol-1; H2 (g) : 28.84 kJ
mol-1; O2 (g) : 29.37 kJ mol-1.
Assume heat capacities are independent of T.
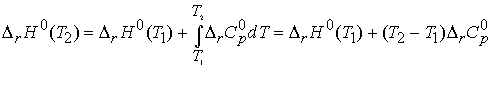

Chapter 3 First Law: The Machinery
The Mathematics of Thermodynamics:
The power of thermodynamics is in being able to relate measurable
properties to those that are not so easily measured. Many of the relationships involve the slopes of functions, the
rate of change of one variable with respect to another, the derivative. To understand how these relationships are
derived we need to understand some mathematical properties of differentials and
partial derivatives. See Further
Information 1, pg. 905-906.
Exact differentials
Recall that change in internal energy, dU, is called an exact differential because it depends only on initial and final state of the system but not the path. We will define exact differential mathematically.
If we have a function arbitrarily designated by J which depends upon the variables T and p so that we may write J = J(T, p), then the infinitesimal change in J, dJ, is given by
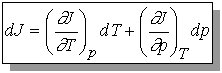
The variables T and p must be independent and J(T,p) must be
continuous, single-valued, differentiable.
(no step functions, one value of J for every T and p, no functions
with cusps)
The partial derivatives will, in general, also be functions of T
and p, derivable from J(T,p) by standard differentiation techniques. We could
therefore define the functions M and N by
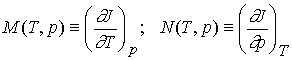
The expression for dJ becomes
dJ = MdT + Ndp
We
know that the order of differentiation doesn’t matter for second
derivatives.
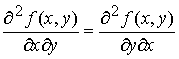
If the original function J(T,p) is continuous, single-valued and
differentiable, then
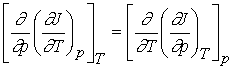
Inserting
M and N for their differentials