|
Zooplankton of the
Great Lakes <Home> |
Site
created by: Doug Larson
|
|
Classification and Life
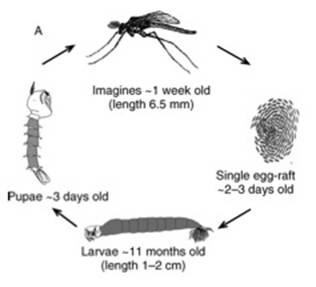
History Kingdom: Animalia Phylum: Arthropoda Class: Insecta Order: Diptera
Family: Chaoboridae Genus: Chaoborus Chaoborus, also known as The Phantom Midges, are a
predacious omnivore that develops sequentially in both aquatic and terrestrial
zones in three stages. The first two of these three stages occur in aquatic
environments (Diomande et al. 2010). The majority of the life cycle is spent
in the larval stage, which can vary from seven days to several months
(Diamande et al. 2010; Berendonk et al. 2009; VonEnde 1982), while the
nymphal stage will last only 2-4 days (Diamande et al. 2010). The imago stage
of development occurs in the air, and generally lasts for less than 10 days
(Diamande et al. 2010) (Figure 1). Some species of Chaoborus (C. punctipennis) have been seen to have two generations per
year, one overwintering-spring generation and a summer generation (Eaton
1983).
Figure 1. Development stages of Chaoborus sp. from egg to adult (Berendonk et al. 2009). Habitat Selection and Behavior Many zooplankton species will exhibit a behavior called Diel
Vertical Migration (DVM). This occurs when a zooplankton is near the bottom
of the water column during the daytime, and migrates to the surface near dusk
and into the nighttime. Zooplankton may migrate vertically as a result of
light, prey abundance, turbidity, and temperature. Larval Chaoborus exhibit DVM in the presence
of fish (Stall 1966; Northcote 1964). Interestingly, in the absence of fish Chaoborus do not exhibit DVM
(Northcote 1964), which suggests that Chaoborus
are able to detect chemical cues (kairomones) from
fish and can also detect their absence. Feeding Ecology Chaoborus are often consider opportunistic eaters, as
they will eat both copepods and cladocerans (Pastorok 1980; Swift and Fedorenko 1975). However,
in a laboratory setting, given the choice between the two, Chaoborus prefers the copepods to the
cladocerans (Pastorok 1980). In the case of Diaptomus and Daphnia,
Pastorok (1980) suggests that this difference may result from the much higher
swimming rate of Daphnia. When
resources are limited, or copepods are not present, Chaoborus will select
Daphnia as an adequate substitute. In most cases, Chaoborus selects the prey that enters it effective stick zone,
and does not tend to chase its prey (Swift and Fedorenko 1975). Another
factor in feeding is head size. As mandible and head size vary from species
to species, prey selection will also vary (Swift and Fedorenko 1975). |
Anatomy While many zooplankton
exhibit DVM, Chaoborus is uniquely
adapted to migrate vertically even when oxygen is not present. While many
zooplaknton require high levels of oxygen to produce ATP, Chaoborus uses an anaerobic malate cycle to derive ATP when oxygen is
deprived at the bottom of eutrophic lakes (Maddrell 1998). The ability to
function in environments that are oxygen deprived allows Chaborus to further escape predation by simply migrating to areas
that fish predators cannot tolerate. The
features of Chaoborus make it easy
to distinguish from other zooplankton. Most species of Chaoborus have a long, skinny body cavity with varying amounts of
pigmentation (Von Ende 1982). The high density of pigment on Chaoborus americanus, for example,
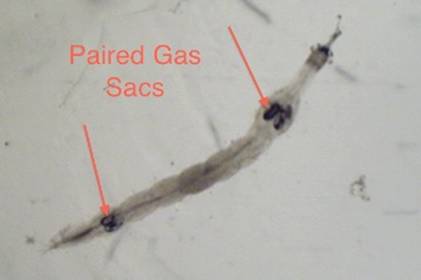
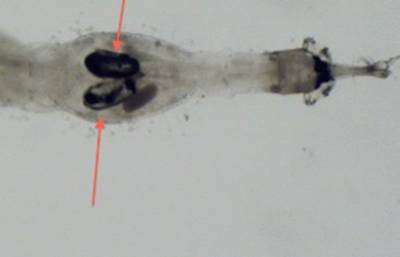
make it better suited for lakes with little to no fish population. Chaborus also features two pair of
darkly pigmented air sacks that are used in migration. The expulsion of gas
results in the sinking of the organism, while the induction of gas will cause
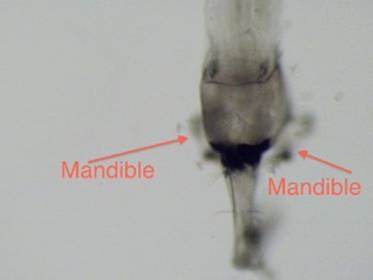
the organism to rise in the water column (Von Ende 1982) (Figures 2, 3). Chaoborus relies mostly on its mandibles for feeding (Figure 4).
The mandibles, which are generally paired, trap and shred prey as they are captured
and pushed back through the digestive system (Weddman and Richter 2007).
Mandibles vary in size based on species (Weddman and Richter 2007).
Figure
2. Both sets of paired gas sacks under 10x magnification.
Figure 3. Paired gas sacks
under 30x magnification.
Figure 4. Head of Chaoborus from top. Manidibles visible
on each side of the head. |
|
|
|
Works Cited:
Berendonk, T. U., Spitze, K.,
and Kerfoot, W.C. (2009). Ephemeral metapopulations show high genetic
diversity at regional scales. Ecology.
90(10): 2670-2675.
Diomande, D., Er, T. T.,
Franquet, E., Maasri, A., Quattara, A., and Gourene, G. (2010). Temporal
dynamics of Chaoborus larvae
(Diptera : Chaoboridae) in the tropical ecosystem. Sciences and Nature 7(1): 51-58.
Eaton, K. A. (1983).
The life history and production of Chaoborus punctipennis
(Diptera: Chaoboridae) in Lake Norman , North Carolina , USA. Hydrobiologia. 106: 247-252.
Fedorenko, A.Y., and Swift,
M.C. (1972) Comparative Biology of Chaoborus Americanus and Chaoborus
Trivittatus in Eunice Lake, British Columbia. Limnology and Oceanography. 17(5): 721-730.
Maddrell, S.H.P. (1998). Why
are there no Insects in the Open Sea? The
Journal of Experimental Biology. 201: 2461-2464.
Northcote, T.G. (1964). Use
of high-frequency echo sounder to record distribution and migration of Chaoborus larvae. Limnology and Oceanography. 9: 8791.
Pastorok, R.A. (1980). The
Effects of Predator Hunder and Food Abundance on Prey Selection by Chaoborus Larvae. Limnology and Oceanography. 25(5): 910-921.
Stahl, J.B. (1966). The
ecology of Chaoborus in Meyers
Lake, Indiana. Limnology and
Oceanography. 11: 177183.
Swift, M.C., and Fedorenko,
A.Y. (1975). Some Aspects of Prey Capture by Chaoborus Larvae. Limnology and Oceanography. 20(3):
418-425.
Von Ende, C. N. (1982).
Phenology of Four Chaoborus Species.
Environmental Entomology. 11(1):
9-15.
Weddman, S and Richter, G.
(2007). The Ecological Role of Immature Phantom Midges (Diptera: Chaoboridae)
in the Eocene Lake Messel, Germany. African
Invertebrates. 48(1): 59-70.
|
|
|
|
|