Review - Important Concepts for Lectures
over Metabolism
I assume that you have had an
introduction to the basics of metabolism in an introductory biology course. The
metabolism you learned was probably entirely focused on the types of metabolism
that animal (maybe plant) cells carry out --aerobic respiration; perhaps you
were exposed to lactic acid fermentation (When muscles are working very hard,
they may be temporarily depleted of oxygen, muscle cells can perform lactic
acid fermentation for a short period of time. The lactic acid end products are
secreted by the muscle cells into your tissues, and you feel the lactic acid as
muscle soreness). The microorganisms are
tremendously more diverse and complex in metabolic patterns than are Eucarya and I want to spend our time emphasizing what
microbes can do, not just covering what you have already had in other courses.
So, if you do not remember
the basics of metabolism you will need to review. The following pages should
serve as a reminder. If it doesnt all come back to you then read Chapter 5 in
the text. If you have not had chemistry
you will also need to read Chapter 2.
Review of oxygen tolerance:
·
Obligate anaerobe does not require O2.
·
Aerotolerant anaerobe does not require O2 for .
·
Microaerophile needs a little O2 for
metabolism, but less than amount present in the atmosphere.
·
Facultative anaerobe can switch its metabolism based on whether or not
O2 is present.
·
Aerobe (obligate aerobe) requires O2 for metabolism.
Review of nutritional patterns:
Source of energy Source
of carbon
Chemicals CO2 (used by autotrophs)
organic Organic molecules
(-C-C-C-) (used by heterotrophs)
inorganic
Light
Most common combinations of
Energy gaining strategy plus Carbon gaining strategy
Chemoorgano heterotrophs
Chemolitho autotrophs
Photo autotrophs
Photo
heterotrophs
You should also know
Definitions of metabolism,
anabolism, and catabolism
That ATP (Adenosine Tri
Phosphate) is made to store energy and used to release energy it is the
energy currency for the cell.
Pyruvate is a key intermediate molecule in many catabolic
pathways.
Should understand basics of oxidations - reductions
Remember - A loss of an electron is called an oxidation;
a gain of an electron is called a reduction (remember as: LEO the lion
says GER).
In biological molecules it is
usually the entire H atom (electron and proton) that is lost or gained, but not
always. Sometimes the electrons are separated from the proton and only the
electrons are lost or gained; and sometimes it may be one H atom + 1 electron
(from a second H atom) that are lost or gained.
In any pair of molecules you
can distinguish which is the oxidized and which is the reduced:
Oxidized state Reduced state:
Contains more oxygen atoms OR Contains fewer oxygen atoms OR
fewer hydrogen atoms AND more hydrogen atoms AND
therefore has fewer electrons and is therefore has more electrons and is
less negative or more positive more
negative or less positive
Example pairs:
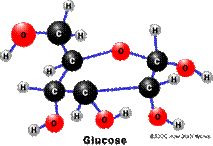
Glucose Pyruvate
C6H12O6 C3H4O3
NAD+ NADH
Sulfate Hydrogen
sulfide
SO4 H2S
All cells need:
1. A source of carbon
for making cellular molecules.
There
are two strategies for obtaining carbon:
a.
Recycle the C already present in some organic (-C-C-) molecule
b.
Use CO2 from the atmosphere
2. A source of energy for performing all cellular work (building
molecules, transport across the plasma membrane, locomotion, etc.)
Energy
is created by harvesting the electrons present in:
|
a. Organic molecules. |
|
(specifically the electrons
in the H atoms in the molecules) Hydrogen showing the proton and electron |
|
like
a sugar or an amino acid OR |
|
|
|
b. Inorganic molecules. electrons
in molecules like |
|
|
|
|
ammonia |
hydrogen sulfide |
|
The more electrons a
molecule has, the more energy the molecule is capable of yielding so look
at glucose compared to hydrogen sulfide which molecule should yield the
most energy? (glucose 12 H vs. 2 in
H2S) |
||
|
The electrons that are
released when bonds are broken have to go somewhere, so they get passed from
the donor (the molecule that you started with that had all the electrons) to
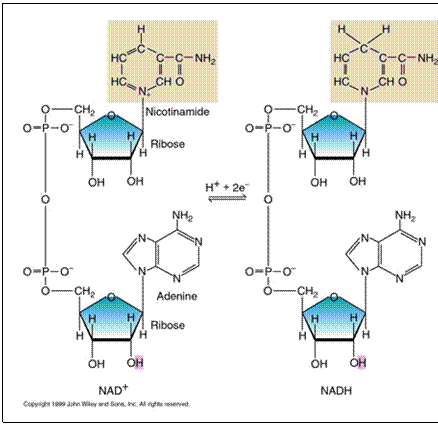
intermediate electron carriers. NAD+ is a
soluble carrier present in the cytoplasm. It is lacking 1 electron (1 H) and
so it can accept 1 electron (1 H). As it accepts the electron, it is reduced
to NADH. |
|
|
|
|
Oxidized state fewer H, fewer e- more positive (NAD+) |
Reduced state more H, more e- |

|
NAD+ is in
limiting quantities in the cell and it must be regenerated if energy
production is to continue. There are 2 ways to
regenerate NAD+ from NADH :
|
|||
|
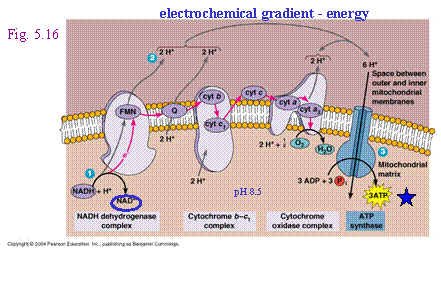
2. NADH travels to the cytoplasmic
membrane and passes the electron off to the electron transport chain. This
process is called respiration. (NADH
then becomes NAD+ ) |
|
||
|
The electrons are passed
along the chain, generating two types of usable energy along the way
electrochemical gradient and ATP - until they reach a final electron
acceptor, an inorganic molecule which
can be: |
||
|
a.
oxygen (aerobic respiration) OR |
|
As oxygen accepts electrons it will become reduced
to H2O
|
|
b. some other inorganic molecule (anaerobic
respiration) |
like nitrate
|
or sulfate
|
|
|
becomes reduced to nitrite (NO2) |
becomes reduced to hydrogen sulfide (H2S) |
Note fermentation is NOT anaerobic
respiration. By definition respiration requires both an electron transport
chain and an inorganic terminal electron acceptor. Fermentation does not employ
an electron transport chain and the terminal electron acceptor is an organic
molecule. Fermentation takes place in the absence of oxygen, it can occur in
anoxic and anaerobic environments, but it is not respiration!
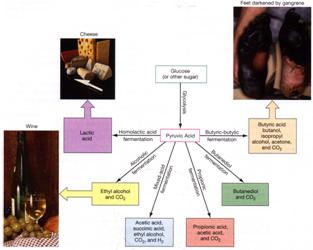
Comparison of Respiration vs Fermentation in Chemoorganotrophs
|
|
Respiration |
Fermentation |
|
|
Initial electron donor: |
organic molecule |
organic molecule |
|
|
examples: |
carbohydrates, amino acids, lipids |
carbohydrates, amino acids, lipids |
|
|
Intermediary electron carrier(s): |
NADH, FADH2, carriers in the electron
transport chain |
NADH |
|
|
Final electron acceptor |
inorganic molecule |
organic molecule |
|
|
examples: |
O2 |
CO2, NO3, SO4 |
pyruvate |
|
final
electron acceptor reduced to: |
H2O |
CH4, NO2, H2S |
lactic acid, acetic acid, ethanol, etc. |
|
example
organisms |
Mitochondria, E. coli, Pseudomonas, S. aureus |
Methanogens, E. coli, Pseudomonas,
Sulfate-reducing bacteria |
Bifidobacterium,
Lactobacillus, E. coli, Clostridium, Bacteroides |
|
Potential net ATP yield: |
as many as 38 if starting with 1
glucose by aerobic respiration with an electron transport chain containing
all the cytochromes but often far fewer than 38 -
but still more than 2. |
2 |
|
Comparison of Respiration in Chemoorganotrophs vs Chemolithotrophs
|
|
Chemoorganotroph |
Chemolithotroph |
|
|
Initial electron donor: |
organic (-C-C-) molecule |
inorganic molecule |
|
|
examples: |
carbohydrates, amino acids, lipids |
hydrogen gas, ammonia, nitrate, hydrogen sulfide |
|
|
Electron donor oxidized to: |
CO2 |
water, nitrate, nitrite, sulfuric acid |
|
|
Final electron acceptor |
inorganic molecule |
inorganic molecule |
|
|
examples: |
O2 (aerobic respiration) |
CO2, NO3, SO4
(anaerobic respiration) |
O2 (aerobic respiration) |
|
electron
acceptor reduced to: |
H2O |
CH4, NO2, H2S |
H2O |
|
example
organisms |
Mitochondria, E. coli, Pseudomonas, S. aureus |
Methanogens, E. coli,
Pseudomonas, Sulfate-reducing
bacteria |
Alcaligenes, Nitrosomonas, Nitrobacter, Thiomargarita |